Main Article Content
Abstract
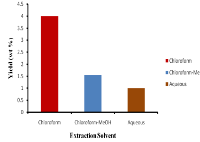
Medical professionals have used synthetic medicine to treat bacterial infections. Scientists globally have been on the search for the utilization of natural plant products as alternatives. Therefore, this study investigated the preliminary phytochemical screening, and characterization of bioactive compounds and antibacterial properties of Eupatorium odoratum. Phytochemicals were detected using standard methods, certain bioactive compounds were detected using thin layer chromatographic (TLC) technique, ultra-violet (UV) visible and Fourier transform infra-red (FTIR) spectroscopic techniques. Antimicrobial screening was carried out using the punched agar diffusion method, the minimum inhibitory concentration and minimum bactericidal concentration of test organisms were determined by using serial doubling dilution method. Saponins, tannins, glycosides, alkaloids, steroids, terpenoids, proteins, oil were detected in the crude extract and some bioactive compounds which denoted the presence of various phytochemicals were found in the three solvent extracts used. All extracts showed good antibacterial activities with zone inhibition of 15-25 mm against all microorganism specimens; however, the chloroform extract was more potent in causing inhibition with Staphylococcus aureus (25 mm), having the highest zone of inhibition. Eupatorium odoratum can therefore be utilized as an alternative medicinal agent against a variety of bacterial infections.
Keywords
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
- Vaisakh M N, Pandey A. Pharmacognostic study of leaves of Chromolaena odorata UNM. Intl J Pharmaceu Res Dev. 2012a; 4(2): 33-37.
- Aziz NA, Mohamad M, Mohsin HF, Nor Hazalin NA M, Hamid KA. The pharmacological properties and medicinal potential of Chromolaena odorata: A Review. Intl J Pharmaceu Nutraceu Cos Sci. 2020; 2: 30-34.
- Onakurhefe P, Onyeukwu OB, Ohwokevwo OA, Achuba FI. Effect of methanolic extract of Justicia flava leaves on biochemical markers
- in male Wistar rats fed crude oil contaminated feed. J Appl Sci Environ Manag. 2022; 26(10):1689-1694.
- Onyeukwu OB, Dibie DC, Njideaka OT. Hibiscus sabdariffa - uses, nutritional and therapeutic benefits - A review. J Biosci Biotechnol Discov. 2023; 8(2):18-23.
- Kilani AM. Antibacterial assessment of whole stem bark of Vitex doniana against some Enterobactriaceae. Afric J Biotechnol. 2006; 5: 958-959.
- Sukanya SL, Sudisha J, Hariprasad P, Niranjana SR, Prakash HS, Fathima SK. Antimicrobial activity of leaf extracts of Indian medicinal plants against clinical and phytopathogenic bacteria. Afric J Biotechnol. 2009; 8(23): 6677-6682.
- Yazdi FT, Behbahani BA, Mortazavi A. Investigating the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the Lavandula stoechas L. and Rosmarinus officinalis L. extracts on pathogen bacterias “in vitro”. J Paramed Sci. 2014; 5(2): 91- 101.
- Prawiradiputra BR. Ki Rinyuh (Chromolaena odorata (L) R M King dan H Robinson: Gulma padang rumput yang merugikan. Wartazoa, 2007; 17(1): 46-52
- Patel J, Kumar GS, Qureshi MS, Jena PK. Anthelmintic activity of ethanolic extract of whole plant of Eupatorium odoratum. L. Intl J Phytomed. 2010; 2: 127-132.
- Chakraborty AK, Rambhade S, Patil UK. Chromolaena odorata (L.): an overview. J Pharm Res. 2011; 4(3): 573-576.
- Vaisakh MN, Pandey A. The invasive weed with healing properties: A review on Chromolaena odorata. Intl J Pharmaceu Sci. 2012b; 3(1):80–3.
- Devi GB, Ramya KS, Sri DS, Josthna P, Naidu CV. Phytochemical screening study in different parts of Chromolaena odorata by LC MS method and related parameters. Intl J Sci Res Arch. 2022; 07(02): 128–140.
- Nwachukwu I, Aliga C, Upabi CF, Ukogo I. Invitro antibacterial effect of crude extract of Chromolaena odorata leaves on wound isolates. IOSR J Pharm Biol Sci. 2016; 11(6): 49–52.
- Omokhua AG, Mcgaw LJ, Chukwujekwu JC, Finnie JF, VanStaden J. A comparison of the antimicrobial activity and in vitro toxicity of a medicinally useful biotype of invasive Chromolaena odorata (Asteraceae) with a biotype not used in traditional medicine. South Afri J Bot. 2017; 108: 200–208.
- Putri DA, Fatmawati S. A new flavanone as a potent antioxidant isolated from Chromolaena odorata l. leaves. EvidenceBased Comp Alter Med. 2019; 1453612.
- Vijayaraghavan K, Rajkumar J, Seyed MA. Efficacy of Chromolaena odorata leaf extracts for the healing of rat excision wounds. Vet Med. 2017; 62(10): 565–578.
- Sirinthipaporn A, Jiraungkoorskul W. Wound healing property review of Siam weed, Chromolaena odorata. Pharmacog Rev. 2017; 11(21):35-38.
- Rojas R, Bustamante B, Bauer J. Antimicrobial activity of selected Peruvian medicinal plants. J Ethanopharm. 2003; 88: 199-204.
- Bereksi MS, Hassaïne H, Bekhechi C, Abdelouahid D E. Evaluation of antibacterial activity of some medicinal plants extracts commonly used in Algerian traditional medicine against some pathogenic bacteria. Pharmacog J. 2018; 10(3):507-12.
- Tiwari S, Nepal S, Sigdel S, Bhattarai S, Rokaya RK, Pandey J, Khadka RB, Aryal P, Bhandari R. Phytochemical screening, antibacterial-guided fractionation, and thinlayer chromatographic pattern of the extract obtained from Diploknema butyracea. Pharmacog Res. 2020; 12:437-43.
- Borokini TI, Omotayo FO. Comparative phytochemical analysis of selected medicinal plants in Nigeria.International J Adv Chem Res. 2012; 1(1): 011-018.
- Njoku OV, Chidi O. Phytochemical constituents of some selected medicinal plants. Afri J Pure Appl Chem. 2009; 3(11): 228-233.
- Kwekowe CG, Johnbull EO, Otuokere IE. Isolation and characterization of secondary metabolite from the stem bark extract of Allophylus africanus Beauv (Sapindaceae). J Chem Soc Nig. 2021; 46(2): 0382 – 0392.
- Aye MM, Noe Oo WM. Screening of some bioactive constituents from the bark of Cinchona succirubra pav. (kwi-neing). J Myan Acad Arts Sci. 2019; 17(1A): 27-39.
- Yazdi FT, Behbahani BA, Mortazavi A. Investigating the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of the Lavandula stoechas L. and Rosmarinus officinalis L. extracts on pathogen bacterias “in vitro”. J Paramed Sci. 2014; 5(2): 91-101
- Islam S, Rahman A, Sheikh MI, Rahman M, Jamal AHM, Alan F. In vitro antibacterial activity of methanol seed extract of Elettaria cardamomum (L.) Maton Agric Consp Scient. 2010; 75(3): 113-117.
- Muhamad N, Muhmed SA, Yusoff MM, Gimbun J. Influence of solvent polarity and conditions on extraction of antioxidant, flavonoids and phenolic content from Averrhoa bilimbi. J Food Sci Eng. 2014; 4: 255-260.
- Ahmed SA, Yasser MD. Effect of various extraction methods and solvent types on yield, phenolic and flavonoid content and antioxidant activity of Spathodea nilotica leaves. Egypt J Chem. 2021; 64(12): 7583– 7589.
- Mishra D, Sarkar DK, Nayak BS, Rout PK, Ellaiah P, Ramakrishna S. Phytochemical investigation and evaluation of anthelmintic activity of extract from leaves of Eupatorium odoratum linn. Ind J Pharmaceu Edu Res. 2010; 44(4): 369-374.
- Yusuf H, Fahriani M. Anticancer activity and apoptotic induction of Chromolaena odorata Linn leaves extract and fractions on hepatocellular carcinoma cell lines (HepG2). J Nat. 2022; 22(1): 57-67.
- Rashmi TS. Phytochemical standardization of Diploknema butyracea (Roxb.) H. J. Lam. seeds by HPLC technique. Ind J Nat Prod Resourc. 2015; 6:299‑304.
- Anand U, Jacobo-Herrera N, Altemimi A,Lakhssassi N. A comprehensive review on medicinal plants as antimicrobial therapeutics: potential avenues of biocompatible drug discovery. Metab. 2019; 9: 258.
- Anand U, Nandy S, Mundhra A, Das N, Pandey DK, Dey A. A Review on Antimicrobial Botanicals, Phytochemicals and Natural Resistance Modifying Agents fromApocynaceae Family: Possible Therapeutic Approaches against Multidrug Resistance in Pathogenic Microorganisms. Drug Resist Updates. 2020; 51: 100695.
- Yu Z, Tang J, Khare T, Kumar V. The alarming antimicrobial resistance in eskapee pathogens: Can essential oils come to the rescue? Fitoterapia. 2020; 140: 104433.
- Mohammed MJ, Anand U, Altemimi AB, Tripathi V, Guo Y, Pratap-Singh A. Phenolic composition, antioxidant capacity and antibacterial activity of white wormwood (Artemisia herba-alba). Plants. 2021; 10: 164.
- Ekanayake S, Jansz ER, Nair BM. Literature review of an underutilized legume: Canavalia gladiate L. Plant Foods Hum Nutr. 2000; 55(4): 305 – 321.
- Aganbi E, Onyeukwu OB, Avwioroko JO,Tonukari NJ. Effect of fermentation on sensory, nutritional and antioxidant properties of mixtures of aqueous extracts of Hibiscus sabdariffa (zobo) and Raphia hookeri (raffia) wine. Nig J Sci Environ. 2017; 15(1): 66 – 74.
- Hassan IA, Nasiru IA, Malut AM, Ibrahim AS, Ali AS. Phytochemical studies and thin layer chromatography of leaves and flower extracts of Senna siamea Lam for possible biomed applications. J Pharmacog Phytother. 2015; 7(3):18‑26.
- Mitchell AM, Mitchell TJ. Streptococcus pneumoniae: virulence factors and variation. Clin Microbiol Infect. 2010; 16: 411–418.
- Sabbagh SC, Forest CG, Lepage C, Leclerc J, Daigle F. So similar, yet so different: uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and Typhi. FEMS Microbiol Lett. 2010; 305:1–13.
- Bachir RG, Abouni B. Escherichia coli and Staphylococcus aureus most common source of infection. In: The battle against microbial pathogens: Basic Science, Technological Advances and Educational Programs, Méndez-Vilas A ed.; Formatex Research Center, Spain, pp. 637-648.
- Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015; 28(3): 603-61.
- Sun Y, Feng JQ, Tan YR, Zhou L, Lan T, Ma JY. Genomic and biological characterization of vB_PvuS_Pm34, a novel lytic bacteriophage that infects Proteus vulgaris. Genom. 2021; 114: 38–44.
- Jai S, Kundu A, Jeyakumar S, De AK. Antimicrobial activities of Eupatorium odoratum leaves. Ind Vet J. 2012; 89(1): 24- 25.
- Natheer SE, Sekar C, Amutharaj P, Rahman MSA, Khan KF. Evaluation of antibacterial activity of Morinda citrifolia, Vitex trifolia and Chromolaena odorata. Afric J Pharm Pharmacol. 2012; 6: 783-788.
- Kumar S, Vaidya A, Pilania P. Alkaloids as efflux pump inhibitors: A positive approach for combating antimicrobial resistance. Biotechnol Rep. 2021; 30: e00610.
- Li C, Scott DA, Lemaître N, Veras G, Diamondk AM, Chiasm E, Zuckerbraun BS, Kaczmarek K, Remick DG, Baron CH. Alkaloids as antimicrobial agents: advances and challenges in therapy. J Med Chem. 2020; 63: 3848-3873.
- Maurya A, Dwivedi GR, Darokar MP,Srivastava SK. Antibacterial and synergy of clavine alkaloid lysergol and its derivatives against nalidixic acid-resistant Escherichia coli. Chem Biol Drug Des. 2013; 81: 484–490.
- Sridevi D, Shankar C, Prakash P, Park J, Thamaraiselvi K. Inhibitory effects of reserpine against Efflux pump activity of antibiotic resistance bacteria. Chem Biol Lett. 2017; 4: 69–72.
- Thawabteh A, Juma S, Bader M, Karaman D, Scrano L, Bufo S, Abualhasan MN, Husein I, Alkader ED, Al-Awabdeh M, Robinson T, Alali F, Alquran L, Petróczi A. The biological activity of natural alkaloids against herbivores, cancerous cells and pathogens. Toxins. 2019; 11: 656.
- Omar F, Tareq AM, Alqahtani AM, Dhama K, Sayeed MA, Emran T, Ali BH, Sharma V. Plant-based indole alkaloids: a comprehensive overview from a pharmacological perspective. Molecules. 2021; 26(8): 2297.
- Anyasor GN, Aina DA, Olushola M, Aniyikaye AF. Phytochemical constituent, proximate analysis, antioxidant, antibacterial and wound healing properties of leaf extracts of Chromolaena odorata. Ann Biol Res. 2011; 2(2): 441-451.
- Lavanya G, Brahmaprakash GP. Phytochemical screening and antimicrobial activity of compounds from selected medicinal and aromatic plants. Intl J Sci Nat. 2011; 2(2): 287-291.
- Akinmoladun AC, Ibukun EC, DanOloge IA. Phytochemical constituents and antioxidant properties of extracts from the leaves of Chromolaena odorata. Scient Res Essays. 2007; 2: 191-194.
- Maji S, Dandapat P, Ojha D, Maity C, Halder SK, Das Mohapatra PK, Mondal KC. In vitro antimicrobial potentialities of different solvent extracts of ethnomedicinal plants against clinically isolated human pathogens. J Phytol. 2010; 2(4): 57-64.
- Kothari V, Gupta A, Naraniwal M. Comparative study of various methods for extraction of antioxidant and antibacterial compounds from plant seeds. J Nat. Remed. 2012; 12(2): 162-173.
References
Vaisakh M N, Pandey A. Pharmacognostic study of leaves of Chromolaena odorata UNM. Intl J Pharmaceu Res Dev. 2012a; 4(2): 33-37.
Aziz NA, Mohamad M, Mohsin HF, Nor Hazalin NA M, Hamid KA. The pharmacological properties and medicinal potential of Chromolaena odorata: A Review. Intl J Pharmaceu Nutraceu Cos Sci. 2020; 2: 30-34.
Onakurhefe P, Onyeukwu OB, Ohwokevwo OA, Achuba FI. Effect of methanolic extract of Justicia flava leaves on biochemical markers
in male Wistar rats fed crude oil contaminated feed. J Appl Sci Environ Manag. 2022; 26(10):1689-1694.
Onyeukwu OB, Dibie DC, Njideaka OT. Hibiscus sabdariffa - uses, nutritional and therapeutic benefits - A review. J Biosci Biotechnol Discov. 2023; 8(2):18-23.
Kilani AM. Antibacterial assessment of whole stem bark of Vitex doniana against some Enterobactriaceae. Afric J Biotechnol. 2006; 5: 958-959.
Sukanya SL, Sudisha J, Hariprasad P, Niranjana SR, Prakash HS, Fathima SK. Antimicrobial activity of leaf extracts of Indian medicinal plants against clinical and phytopathogenic bacteria. Afric J Biotechnol. 2009; 8(23): 6677-6682.
Yazdi FT, Behbahani BA, Mortazavi A. Investigating the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the Lavandula stoechas L. and Rosmarinus officinalis L. extracts on pathogen bacterias “in vitro”. J Paramed Sci. 2014; 5(2): 91- 101.
Prawiradiputra BR. Ki Rinyuh (Chromolaena odorata (L) R M King dan H Robinson: Gulma padang rumput yang merugikan. Wartazoa, 2007; 17(1): 46-52
Patel J, Kumar GS, Qureshi MS, Jena PK. Anthelmintic activity of ethanolic extract of whole plant of Eupatorium odoratum. L. Intl J Phytomed. 2010; 2: 127-132.
Chakraborty AK, Rambhade S, Patil UK. Chromolaena odorata (L.): an overview. J Pharm Res. 2011; 4(3): 573-576.
Vaisakh MN, Pandey A. The invasive weed with healing properties: A review on Chromolaena odorata. Intl J Pharmaceu Sci. 2012b; 3(1):80–3.
Devi GB, Ramya KS, Sri DS, Josthna P, Naidu CV. Phytochemical screening study in different parts of Chromolaena odorata by LC MS method and related parameters. Intl J Sci Res Arch. 2022; 07(02): 128–140.
Nwachukwu I, Aliga C, Upabi CF, Ukogo I. Invitro antibacterial effect of crude extract of Chromolaena odorata leaves on wound isolates. IOSR J Pharm Biol Sci. 2016; 11(6): 49–52.
Omokhua AG, Mcgaw LJ, Chukwujekwu JC, Finnie JF, VanStaden J. A comparison of the antimicrobial activity and in vitro toxicity of a medicinally useful biotype of invasive Chromolaena odorata (Asteraceae) with a biotype not used in traditional medicine. South Afri J Bot. 2017; 108: 200–208.
Putri DA, Fatmawati S. A new flavanone as a potent antioxidant isolated from Chromolaena odorata l. leaves. EvidenceBased Comp Alter Med. 2019; 1453612.
Vijayaraghavan K, Rajkumar J, Seyed MA. Efficacy of Chromolaena odorata leaf extracts for the healing of rat excision wounds. Vet Med. 2017; 62(10): 565–578.
Sirinthipaporn A, Jiraungkoorskul W. Wound healing property review of Siam weed, Chromolaena odorata. Pharmacog Rev. 2017; 11(21):35-38.
Rojas R, Bustamante B, Bauer J. Antimicrobial activity of selected Peruvian medicinal plants. J Ethanopharm. 2003; 88: 199-204.
Bereksi MS, Hassaïne H, Bekhechi C, Abdelouahid D E. Evaluation of antibacterial activity of some medicinal plants extracts commonly used in Algerian traditional medicine against some pathogenic bacteria. Pharmacog J. 2018; 10(3):507-12.
Tiwari S, Nepal S, Sigdel S, Bhattarai S, Rokaya RK, Pandey J, Khadka RB, Aryal P, Bhandari R. Phytochemical screening, antibacterial-guided fractionation, and thinlayer chromatographic pattern of the extract obtained from Diploknema butyracea. Pharmacog Res. 2020; 12:437-43.
Borokini TI, Omotayo FO. Comparative phytochemical analysis of selected medicinal plants in Nigeria.International J Adv Chem Res. 2012; 1(1): 011-018.
Njoku OV, Chidi O. Phytochemical constituents of some selected medicinal plants. Afri J Pure Appl Chem. 2009; 3(11): 228-233.
Kwekowe CG, Johnbull EO, Otuokere IE. Isolation and characterization of secondary metabolite from the stem bark extract of Allophylus africanus Beauv (Sapindaceae). J Chem Soc Nig. 2021; 46(2): 0382 – 0392.
Aye MM, Noe Oo WM. Screening of some bioactive constituents from the bark of Cinchona succirubra pav. (kwi-neing). J Myan Acad Arts Sci. 2019; 17(1A): 27-39.
Yazdi FT, Behbahani BA, Mortazavi A. Investigating the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of the Lavandula stoechas L. and Rosmarinus officinalis L. extracts on pathogen bacterias “in vitro”. J Paramed Sci. 2014; 5(2): 91-101
Islam S, Rahman A, Sheikh MI, Rahman M, Jamal AHM, Alan F. In vitro antibacterial activity of methanol seed extract of Elettaria cardamomum (L.) Maton Agric Consp Scient. 2010; 75(3): 113-117.
Muhamad N, Muhmed SA, Yusoff MM, Gimbun J. Influence of solvent polarity and conditions on extraction of antioxidant, flavonoids and phenolic content from Averrhoa bilimbi. J Food Sci Eng. 2014; 4: 255-260.
Ahmed SA, Yasser MD. Effect of various extraction methods and solvent types on yield, phenolic and flavonoid content and antioxidant activity of Spathodea nilotica leaves. Egypt J Chem. 2021; 64(12): 7583– 7589.
Mishra D, Sarkar DK, Nayak BS, Rout PK, Ellaiah P, Ramakrishna S. Phytochemical investigation and evaluation of anthelmintic activity of extract from leaves of Eupatorium odoratum linn. Ind J Pharmaceu Edu Res. 2010; 44(4): 369-374.
Yusuf H, Fahriani M. Anticancer activity and apoptotic induction of Chromolaena odorata Linn leaves extract and fractions on hepatocellular carcinoma cell lines (HepG2). J Nat. 2022; 22(1): 57-67.
Rashmi TS. Phytochemical standardization of Diploknema butyracea (Roxb.) H. J. Lam. seeds by HPLC technique. Ind J Nat Prod Resourc. 2015; 6:299‑304.
Anand U, Jacobo-Herrera N, Altemimi A,Lakhssassi N. A comprehensive review on medicinal plants as antimicrobial therapeutics: potential avenues of biocompatible drug discovery. Metab. 2019; 9: 258.
Anand U, Nandy S, Mundhra A, Das N, Pandey DK, Dey A. A Review on Antimicrobial Botanicals, Phytochemicals and Natural Resistance Modifying Agents fromApocynaceae Family: Possible Therapeutic Approaches against Multidrug Resistance in Pathogenic Microorganisms. Drug Resist Updates. 2020; 51: 100695.
Yu Z, Tang J, Khare T, Kumar V. The alarming antimicrobial resistance in eskapee pathogens: Can essential oils come to the rescue? Fitoterapia. 2020; 140: 104433.
Mohammed MJ, Anand U, Altemimi AB, Tripathi V, Guo Y, Pratap-Singh A. Phenolic composition, antioxidant capacity and antibacterial activity of white wormwood (Artemisia herba-alba). Plants. 2021; 10: 164.
Ekanayake S, Jansz ER, Nair BM. Literature review of an underutilized legume: Canavalia gladiate L. Plant Foods Hum Nutr. 2000; 55(4): 305 – 321.
Aganbi E, Onyeukwu OB, Avwioroko JO,Tonukari NJ. Effect of fermentation on sensory, nutritional and antioxidant properties of mixtures of aqueous extracts of Hibiscus sabdariffa (zobo) and Raphia hookeri (raffia) wine. Nig J Sci Environ. 2017; 15(1): 66 – 74.
Hassan IA, Nasiru IA, Malut AM, Ibrahim AS, Ali AS. Phytochemical studies and thin layer chromatography of leaves and flower extracts of Senna siamea Lam for possible biomed applications. J Pharmacog Phytother. 2015; 7(3):18‑26.
Mitchell AM, Mitchell TJ. Streptococcus pneumoniae: virulence factors and variation. Clin Microbiol Infect. 2010; 16: 411–418.
Sabbagh SC, Forest CG, Lepage C, Leclerc J, Daigle F. So similar, yet so different: uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and Typhi. FEMS Microbiol Lett. 2010; 305:1–13.
Bachir RG, Abouni B. Escherichia coli and Staphylococcus aureus most common source of infection. In: The battle against microbial pathogens: Basic Science, Technological Advances and Educational Programs, Méndez-Vilas A ed.; Formatex Research Center, Spain, pp. 637-648.
Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015; 28(3): 603-61.
Sun Y, Feng JQ, Tan YR, Zhou L, Lan T, Ma JY. Genomic and biological characterization of vB_PvuS_Pm34, a novel lytic bacteriophage that infects Proteus vulgaris. Genom. 2021; 114: 38–44.
Jai S, Kundu A, Jeyakumar S, De AK. Antimicrobial activities of Eupatorium odoratum leaves. Ind Vet J. 2012; 89(1): 24- 25.
Natheer SE, Sekar C, Amutharaj P, Rahman MSA, Khan KF. Evaluation of antibacterial activity of Morinda citrifolia, Vitex trifolia and Chromolaena odorata. Afric J Pharm Pharmacol. 2012; 6: 783-788.
Kumar S, Vaidya A, Pilania P. Alkaloids as efflux pump inhibitors: A positive approach for combating antimicrobial resistance. Biotechnol Rep. 2021; 30: e00610.
Li C, Scott DA, Lemaître N, Veras G, Diamondk AM, Chiasm E, Zuckerbraun BS, Kaczmarek K, Remick DG, Baron CH. Alkaloids as antimicrobial agents: advances and challenges in therapy. J Med Chem. 2020; 63: 3848-3873.
Maurya A, Dwivedi GR, Darokar MP,Srivastava SK. Antibacterial and synergy of clavine alkaloid lysergol and its derivatives against nalidixic acid-resistant Escherichia coli. Chem Biol Drug Des. 2013; 81: 484–490.
Sridevi D, Shankar C, Prakash P, Park J, Thamaraiselvi K. Inhibitory effects of reserpine against Efflux pump activity of antibiotic resistance bacteria. Chem Biol Lett. 2017; 4: 69–72.
Thawabteh A, Juma S, Bader M, Karaman D, Scrano L, Bufo S, Abualhasan MN, Husein I, Alkader ED, Al-Awabdeh M, Robinson T, Alali F, Alquran L, Petróczi A. The biological activity of natural alkaloids against herbivores, cancerous cells and pathogens. Toxins. 2019; 11: 656.
Omar F, Tareq AM, Alqahtani AM, Dhama K, Sayeed MA, Emran T, Ali BH, Sharma V. Plant-based indole alkaloids: a comprehensive overview from a pharmacological perspective. Molecules. 2021; 26(8): 2297.
Anyasor GN, Aina DA, Olushola M, Aniyikaye AF. Phytochemical constituent, proximate analysis, antioxidant, antibacterial and wound healing properties of leaf extracts of Chromolaena odorata. Ann Biol Res. 2011; 2(2): 441-451.
Lavanya G, Brahmaprakash GP. Phytochemical screening and antimicrobial activity of compounds from selected medicinal and aromatic plants. Intl J Sci Nat. 2011; 2(2): 287-291.
Akinmoladun AC, Ibukun EC, DanOloge IA. Phytochemical constituents and antioxidant properties of extracts from the leaves of Chromolaena odorata. Scient Res Essays. 2007; 2: 191-194.
Maji S, Dandapat P, Ojha D, Maity C, Halder SK, Das Mohapatra PK, Mondal KC. In vitro antimicrobial potentialities of different solvent extracts of ethnomedicinal plants against clinically isolated human pathogens. J Phytol. 2010; 2(4): 57-64.
Kothari V, Gupta A, Naraniwal M. Comparative study of various methods for extraction of antioxidant and antibacterial compounds from plant seeds. J Nat. Remed. 2012; 12(2): 162-173.